See how
MIEBO works
The MIEBO difference
MIEBO contains only ONE ingredient1

Water free

Preservative free

Steroid free

UNIQUE FEATURES

LONG LASTING
Resides in tears for up to 6 hours (PK rabbit study)2,3*
Detection of MIEBO in Tears



QUICK AND EVEN SPREADING
Due to low surface tension1,4,5
The above animations are illustrative of a single drop of MIEBO or saline applied from identical pipettes to a glass eye model.
Formulations containing water may have a brief residence time of 3-5 minutes on the ocular surface and a high surface tension that hinders spreading.5,6
HOW MIEBO WORKS
MIEBO inhibits evaporation by forming an anti-evaporative layer1,7
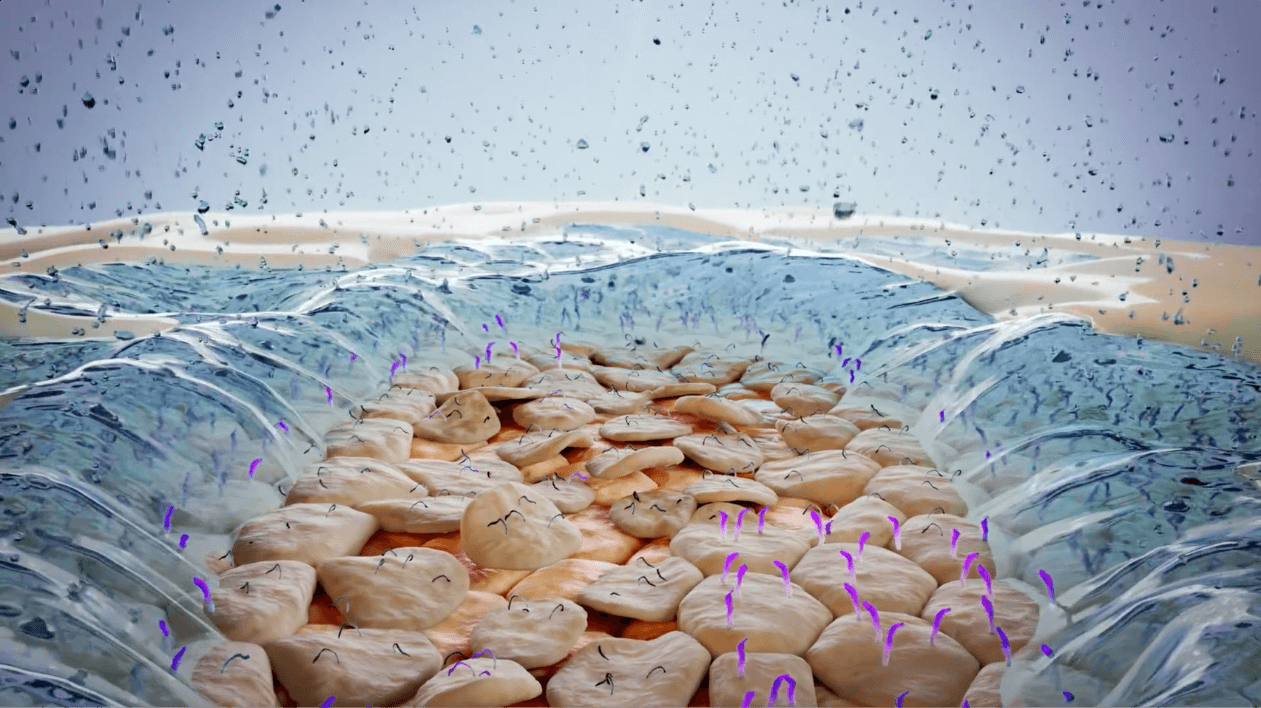
Desiccation and damage of the corneal epithelium2,7
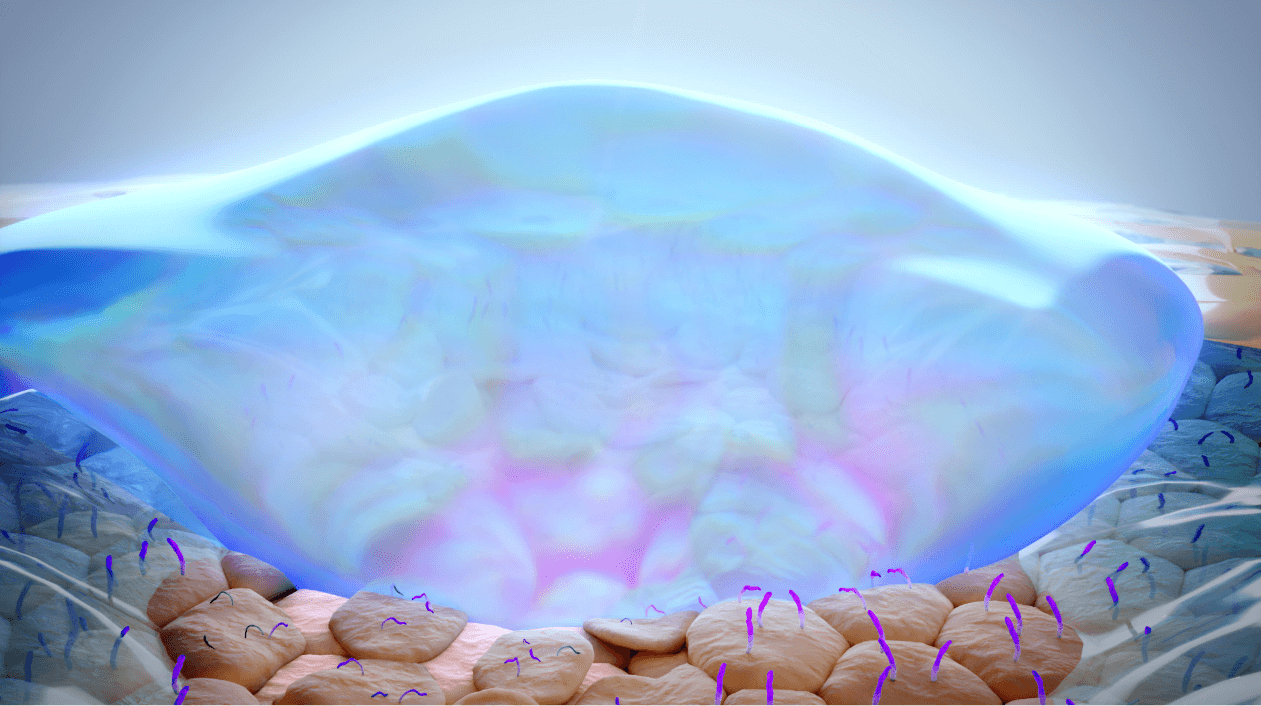
MIEBO spreads across the surface of the eye2

Mimics key functions of natural meibum2,7

Promotes healing on the ocular surface1,7

May reduce friction8-10
MIEBO forms a monolayer at the air-liquid interface of the tear film, which can be expected to reduce evaporation1†
In an in vitro study, MIEBO was shown to be 4x more effective at inhibiting evaporation compared with meibum lipids7‡
‡Study design: The inhibitory effect of MIEBO vs meibum on the evaporation rate (Revap) of saline was evaluated in an in vitro model. Meibum lipids were collected from a single healthy volunteer. The Revap of saline was measured gravimetrically after layering either a single drop of MIEBO or the collected human meibum lipids to approximate the tear lipid layer in vivo over the top of 1 mL saline in a plastic container with a surface area approximating that of the human ocular surface. The Revap of saline was inhibited by 28% when a drop of MIEBO was layered over the top, while a layer of human meibum inhibited the Revap of saline by 8% (P<0.0001 vs control for both). The clinical significance of this in vitro data has not been established.7
HOW MIEBO WORKS
WATCH VIDEO†The exact mechanism of action for MIEBO in DED is not known.1
INDICATION
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
- MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO
- Instruct patients to instill one drop of MIEBO into each eye four times daily
- The safety and efficacy in pediatric patients below the age of 18 have not been established
- The most common ocular adverse reaction was blurred vision (1% to 3% of patients reported blurred vision and conjunctival redness)
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full Prescribing Information for MIEBO.
References: 1. MIEBO. Prescribing Information. Bausch & Lomb, Inc; 2023. 2. Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. Ophthalmol Ther. 2023;12(3):1397-1418. doi:10.1007/s40123-023-00669-1 3. Krösser S, Spencer E, Grillenberger R, Struble CB, Eickhoff K. Ocular and systemic distribution of 14C-perfluorohexyloctane following topical ocular administration to rabbits. Invest Ophthalmol Vis Sci. 2018;59(9):2656. 4. Meinert H, Roy T. Semifluorinated alkanes – a new class of compounds with outstanding properties for use in ophthalmology. Eur J Ophthalmol. 2000;10(3):189-197. doi:10.5301/EJO.2008.1838 5. Agarwal P, Khun D, Krösser S, et al. Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics. Ocul Surf. 2019;17(2):241-249. doi:10.1016/j.jtos.2019.02.010 6. Ahmed S, Amin MM, Sayed S. Ocular drug delivery: a comprehensive review. AAPS PharmSciTech. 2023;24(2):66. doi:10.1208/s12249-023-02516-9 7. Vittitow J, Kissling R, DeCory H, Borchman D. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98:100704. doi:10.1016/j.curtheres.2023.100704 8. Schmidl D, Bata AM, Szegedi S, et al. Influence of perfluorohexyloctane eye drops on tear film thickness in patients with mild to moderate dry eye disease: a randomized controlled clinical trial. J Ocul Pharmacol Ther. 2020;36(3):154-161. doi:10.1089/jop.2019.0092 9. Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516-524. doi:10.1016/j.ophtha.2022.12.021 10. Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE study. Am J Ophthalmol. 2023;252:265-274. doi:10.1016/j.ajo.2023.03.008
INDICATION AND IMPORTANT
SAFETY INFORMATION
INDICATION
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
- MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO
- Instruct patients to instill one drop of MIEBO into each eye four times daily
- The safety and efficacy in pediatric patients below the age of 18 have not been established
- The most common ocular adverse reaction was blurred vision (1% to 3% of patients reported blurred vision and conjunctival redness)
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Click here for full prescribing Information for MIEBO.

