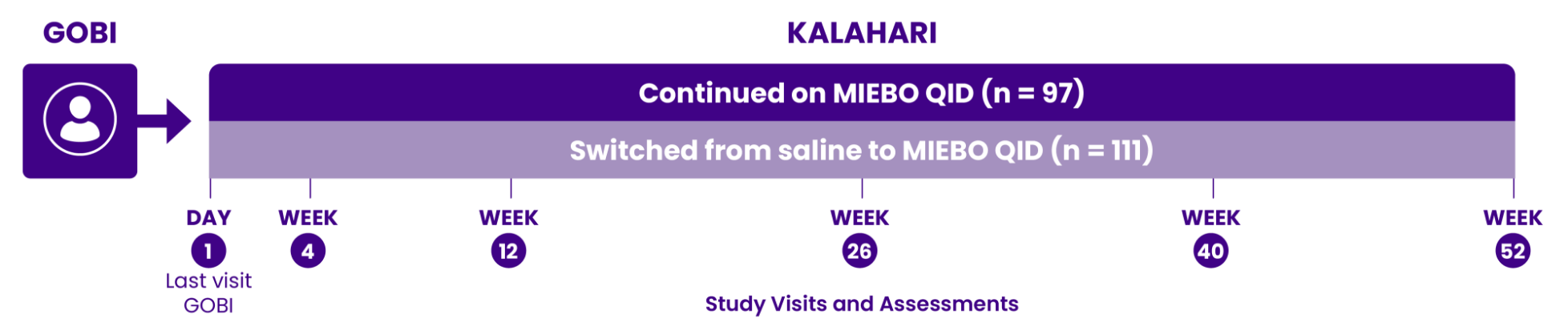
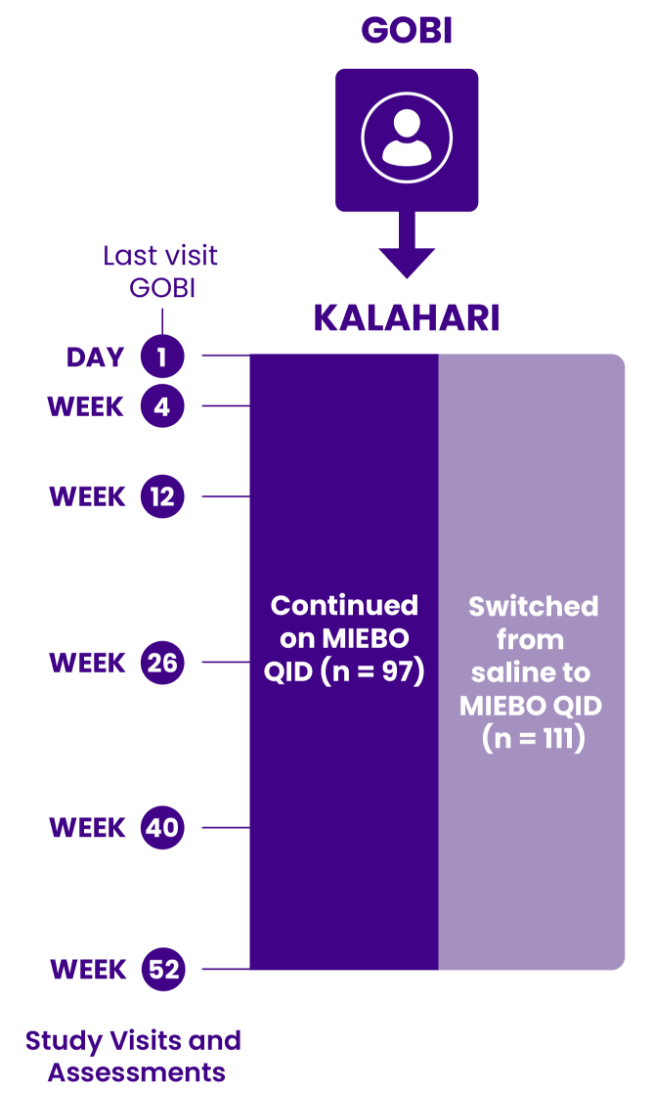
GOBI and MOJAVE study design—100% of
patients had evidence of evaporative DED1-3
The only approved Rx eye drop that required all patients in pivotal studies to
have clinical signs of MGD and TFBUT ≤5 seconds at enrollment1-3
Two 57-day, multicenter, double-masked, saline-controlled studies (GOBI and MOJAVE) were conducted in adults ≥18 years old with a self-reported history of DED in both eyes. Primary outcomes were change from baseline in tCFS and change from baseline in eye dryness score (Visual Analog Scale) at Day 57. Day 15 was the earliest time point at which signs and symptoms were evaluated in the trials. Day 57 was the last.1-3
 SIGN IMPROVEMENT
SIGN IMPROVEMENT
 SYMPTOM RELIEF
SYMPTOM RELIEF
 SIGN IMPROVEMENT-two
SIGN IMPROVEMENT-two

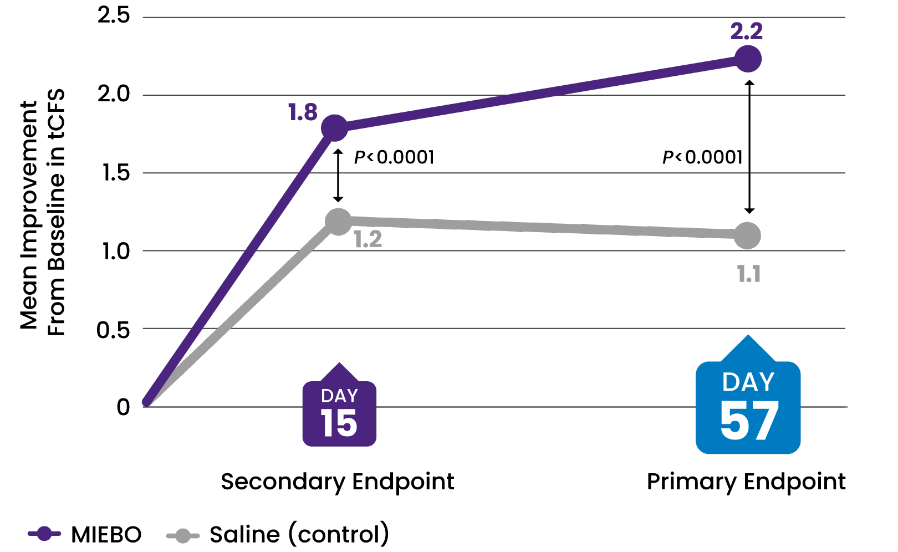

![32% IMPROVEMENT from baseline in tCFS at Day 57 (control, 16%)[2-4]](/siteassets/img/img-216x216-circle-callout-tcfs2x.png)
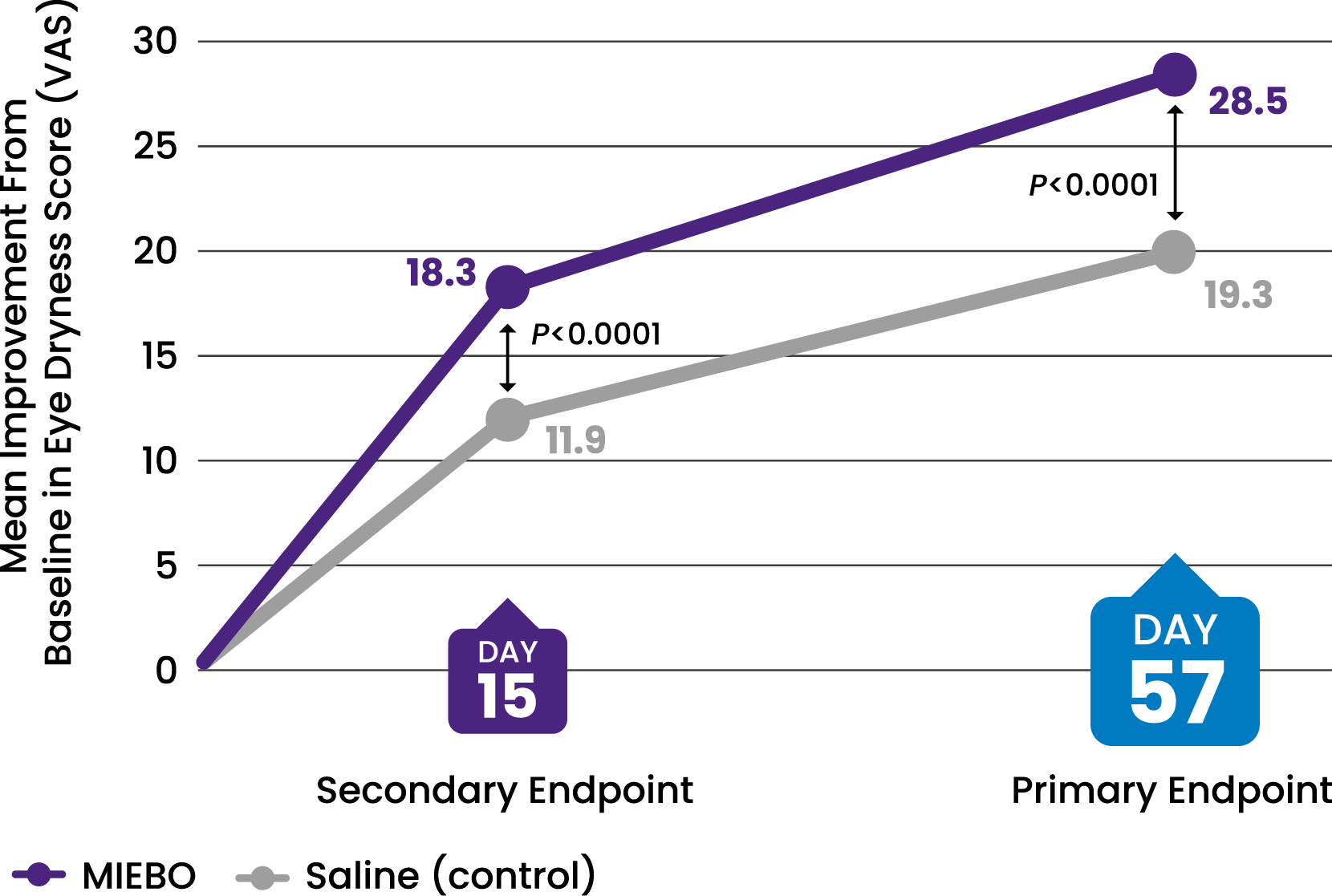

![43% IMPROVEMENT from baseline in eye dryness at Day 57 (control, 29%)[2-4]](/siteassets/img/img-216x216-circle-callout-eye-dryness32.png)
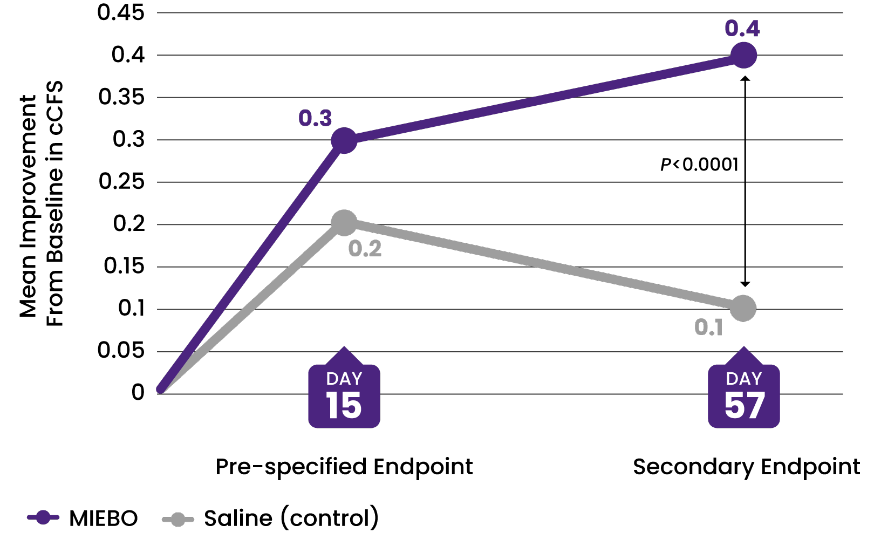

![36% IMPROVEMENT from baseline in cCFS at Day 57 (control, 9%)[2-4]](/siteassets/img/img-216x216-circle-callout-ccfs.png)