Visual Analog Scale Score
PRIMARY ENDPOINT
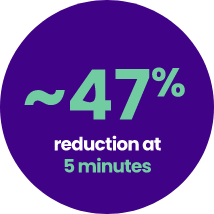
Relief in as fast as 5 minutes with reduced symptoms at every time point8
Patient-reported severity of overall dry eye symptoms


BL, baseline; CFB, change from baseline; SD, standard deviation.
During the 14-day study, MIEBO was dosed QID per label.
Data missing for 1 patient at Day 1 (60 minutes post-dose assessment; item left blank) and 1 patient at Days 7 and 14 (due to study discontinuation).
Patients rated the severity of overall dry eye symptoms on a VAS from 0 (none) to 100 (worst severity possible). Mean (SD) VAS ratings of symptom severity were 72.1 (17.0) at baseline, 38.5 (22.5) at 5 minutes post-instillation on Day 1, 31.7 (22.1) at 60 minutes post-instillation on Day 1, 33.2 (25.1) at Day 3, 27.8 (22.3) at Day 7, and 24.7 (23.0) at Day 14. P<0.0001 vs baseline (paired t test).
Significant reductions in 8 leading symptoms8
Patients rated the severity of the 8 symptoms on a VAS from 0 (none) to 100 (worst severity possible). Across symptoms, percent reduction from baseline severity ranged from 27%-53% at 5 minutes post-instillation on Day 1, 50%-69% at 60 minutes post-instillation on Day 1, 50%-59% at Day 3, 59%-71% at Day 7, and 62%-75% at Day 14. P<0.0001 vs baseline (paired t test) on all item scores at all time points.
Pooled analysis (above): Mean baseline tCFS = 6.9 for MIEBO and saline (control). tCFS grading scale: 0-15 (0-3 in each of 5 areas). Across GOBI and MOJAVE, 614 patients received MIEBO and 603 patients received control with 591 and 575, respectively, assessed on Day 57.2-4
INDIVIDUAL STUDY DATA1-3
GOBI: Mean (SD) CFB –2.0 (2.6) for MIEBO (n = 289) vs –1.0 (2.7) for control (n = 279) (P<0.001) at Day 57.
MOJAVE: Mean (SD) CFB –2.3 (2.8) for MIEBO (n = 302) vs –1.1 (2.9) for control (n = 296) (P<0.001) at Day 57.

 SIGN IMPROVEMENT
SIGN IMPROVEMENT
 SYMPTOM RELIEF
SYMPTOM RELIEF
 SIGN IMPROVEMENT-two
SIGN IMPROVEMENT-two

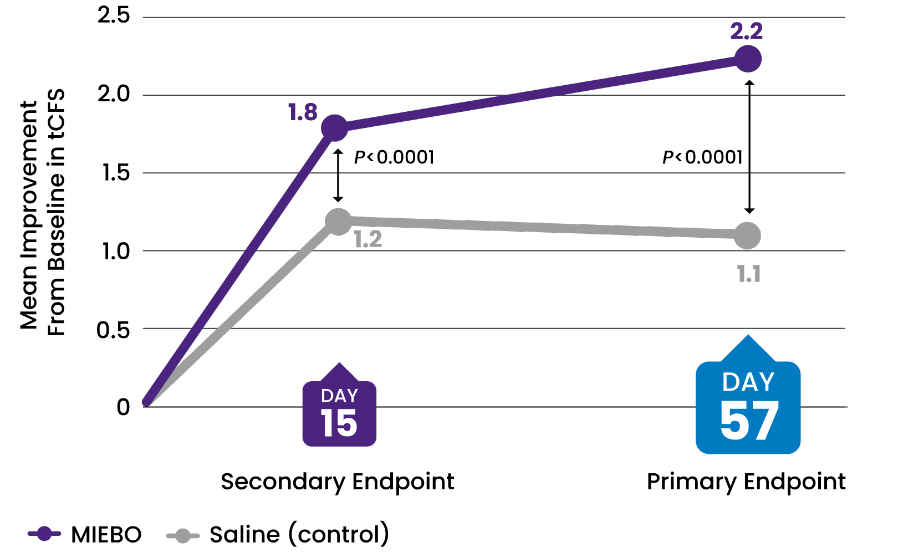

![32% IMPROVEMENT from baseline in tCFS at Day 57 (control, 16%)[2-4]](/siteassets/img/img-216x216-circle-callout-tcfs2x.png)
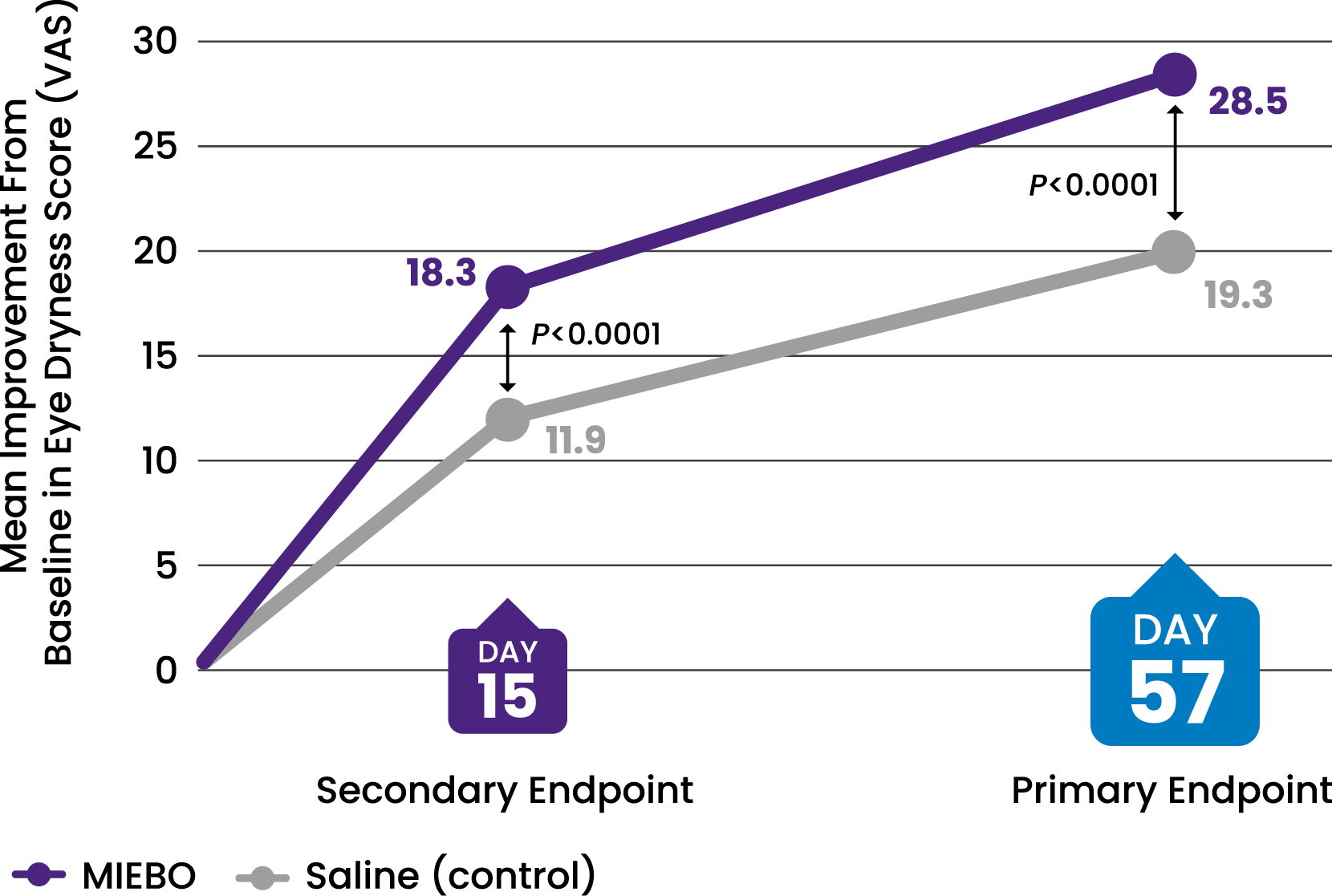

![43% IMPROVEMENT from baseline in eye dryness at Day 57 (control, 29%)[2-4]](/siteassets/img/img-216x216-circle-callout-eye-dryness32.png)
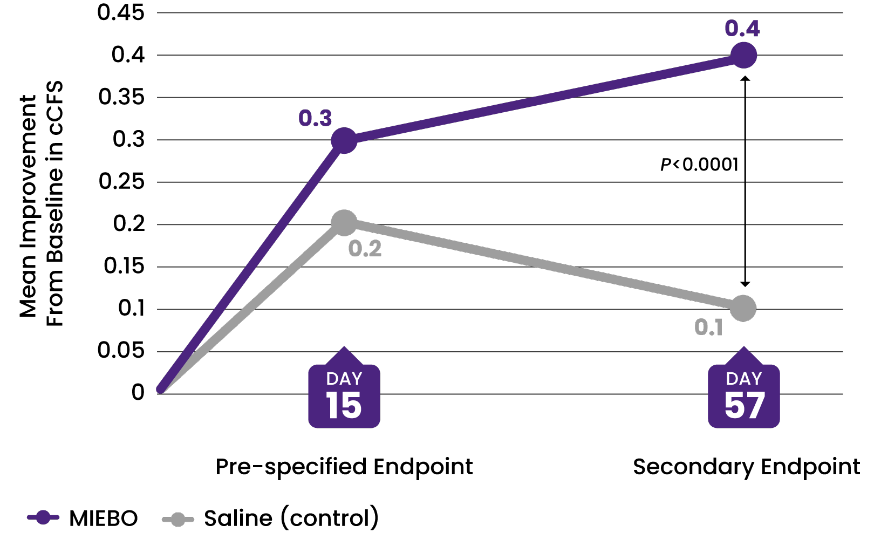

![36% IMPROVEMENT from baseline in cCFS at Day 57 (control, 9%)[2-4]](/siteassets/img/img-216x216-circle-callout-ccfs.png)










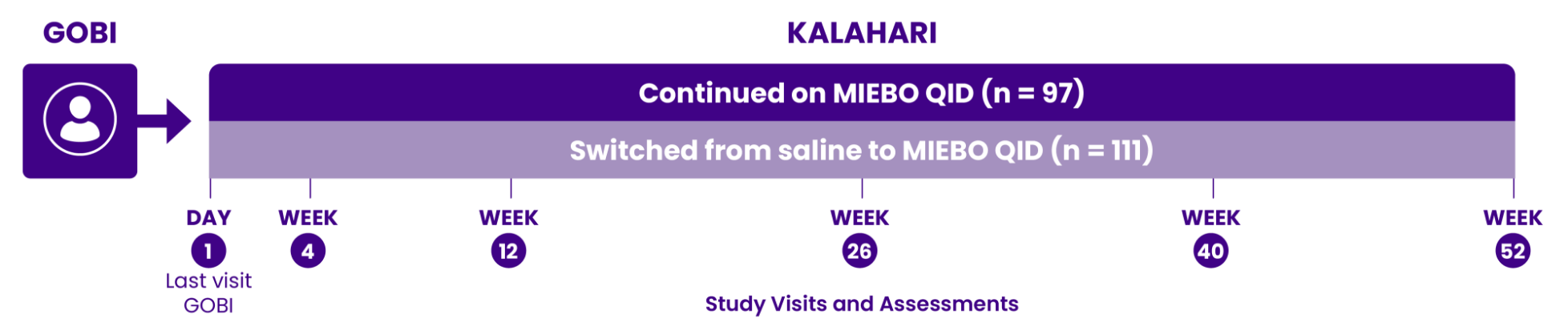
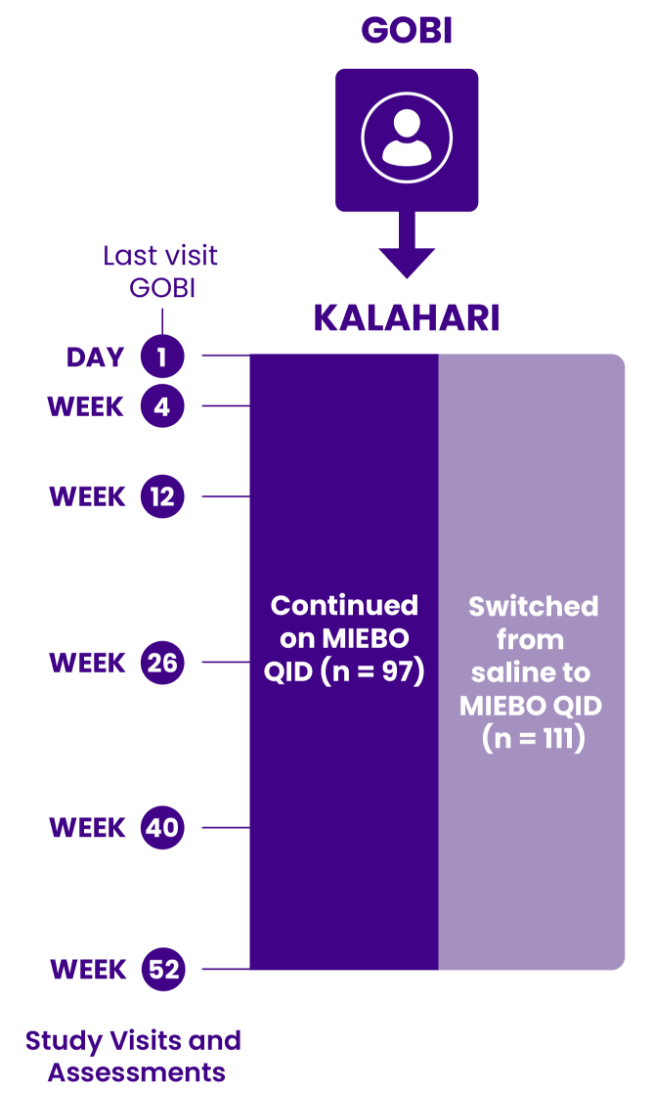
 SYMPTOM REDUCTION
SYMPTOM REDUCTION
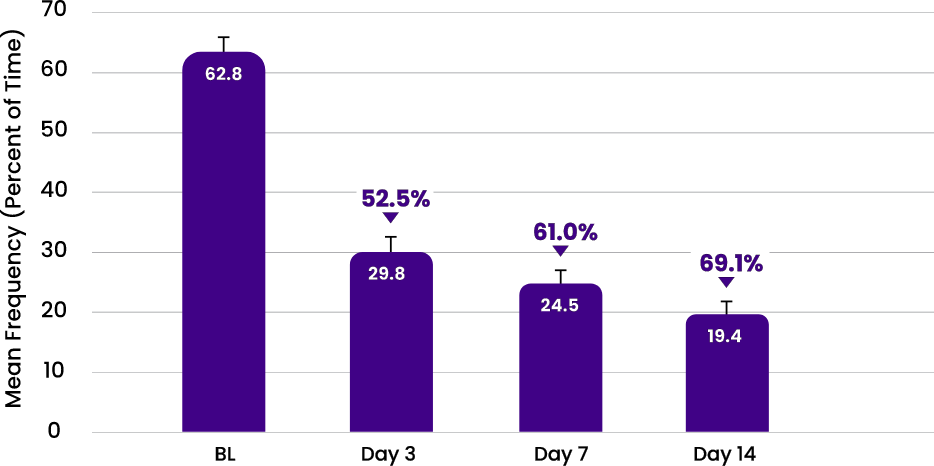
 SYMPTOM FREQUENCY
SYMPTOM FREQUENCY
 OSDI
OSDI